Abstract
Dosing with duvelisib 25 mg BID has been shown to be efficacious in iNHL, most notably the pivotal phase 2 DYNAMO study (NCT01882803) in follicular lymphoma (FL). The TEMPO study (NCT04038359) was designed to examine the effects of prespecified 2-week dose holidays on tumor responses and safety/tolerability in patients with iNHL (FL, MZL), based on data from a phase 3 trial (DUO) in CLL/SLL which demonstrated no negative impacts of (unscheduled) dose interruptions on response.
In TEMPO, patients were randomized to receive duvelisib 25 mg BID for one 10-week cycle followed by 25 mg BID on Weeks 3 and 4 of each subsequent 4-week cycles (Arm 1) or duvelisib 25 mg BID on Weeks 1, 2, 5, 6, 9 and 10 of one 10-week cycle, then on Weeks 3 and 4 of each subsequent 4-week cycles (Arm 2). Patients continued treatment until disease progression, unacceptable toxicity, or withdrawal.
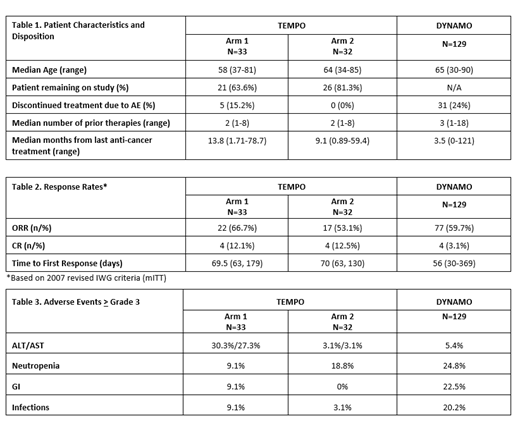
As of May 5, 2021 data cutoff, a total of 65 treated pts had at least 4 months of follow up on or after study drug treatment. Across both arms, the median age was 63 years (range 34-85), and 47 (72.3%) of the patients remained on study treatment at the time of this analysis (table 1). 18 of 65 pts (27.7%) discontinued therapy; 13 of 65 pts (19.7%) discontinued due to PD: 7 out of 33 (21.2%) in arm 1 and 6 out of 32 (18.8%) in arm 2. Of the remaining 5 pts (out of 65 patients) that discontinued, all were in arm 1: 4 out of 33 pts (12.1%) due to adverse events and 1 out of 33 (3%) due to study drug interruption > 42 days. Across both arms, the ORR was 60% (39 of 65 pts), and the CR rate was 12.3% (8 of 65 pts). See table 2 for responses by arm. Grade > 3 AEs were higher in Arm 1 vs Arm 2, with the exception of neutropenia: elevated ALT/AST was 30.3%/27.3% vs 3.1%/3.1%; neutropenia 9.1% vs 18.8%; GI disorders 9.1% vs 0; infections 9.1% vs 3.1%; rash 6.1% vs 3.1%; and drug reaction with eosinophilia 3% vs 0 -- respectively. Discontinuation of study drug due to AEs only occurred in arm 1, in 5 out of 33 patients (15.2%), and were due to ALT/AST elevations (2 of 33 pts, 6.1%); skin and subcutaneous tissue disorders (2 of 33 pts, 6.1%); and pneumonia (1 of 33 pts, 3.0%).
In summary, based on this interim analysis, continuous dosing followed by intermittent dosing (arm 1) and intermittent dosing (arm 2) are both efficacious in patients with iNHL. The ORR and CR are similar to those seen in DYNAMO. The study confirms the overall safety profile of duvelisib, demonstrating that intermittent dosing may well decrease the incidence of side effects without compromising efficacy.
Vorobyev: Janssen, Roche, Sanofi, Takeda, Biocad, Abbvie: Other: Advisory Boards, Speakers Bureau; Astellas, Novartis, AstraZeneca: Speakers Bureau. Tarella: ADC-THERAPEUTICS: Other: ADVISORY BOARD; Abbvie: Other: ADVISORY BOARD. Litwak: Secura Bio: Current Employment. Cohan: Secura Bio: Current Employment. Flinn: Celgene: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Great Point Partners: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; BeiGene: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Agios: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; IGM Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; AbbVie: Consultancy, Other: All Consultancy and Research Funding payments made to Sarah Cannon Research Institute, Research Funding; Verastem: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Curis: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Incyte: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Infinity Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forty Seven: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; ArQule: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Novartis: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Merck: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Constellation Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Unum Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Teva: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Trillium Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; AstraZeneca: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; MorphoSys: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Forma Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Takeda: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Acerta Pharma: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; TG Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Loxo: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Kite, a Gilead Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Nurix Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Roche: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Iksuda Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Janssen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Juno Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Seagen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Rhizen Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Genentech: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Gilead Sciences: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Calithera Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Karyopharm Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Pfizer: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Portola Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Triphase Research & Development Corp.: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Century Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Hutchison MediPharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Vincerx Pharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Sarah Cannon Research Institute: Current Employment; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Seagen: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Unum Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute, Research Funding; Johnson & Johnson: Current holder of individual stocks in a privately-held company; Seattle Genetics: Research Funding. Zinzani: CELLTRION: Other: Advisory board, Speakers Bureau; GILEAD: Other: Advisory board, Speakers Bureau; JANSSEN-CILAG: Other: Advisory board, Speakers Bureau; BMS: Other: Advisory board, Speakers Bureau; TG Therapeutics: Other: Advisory board, Speakers Bureau; TAKEDA: Other: Advisory board, Speakers Bureau; SERVIER: Other: Advisory board, Speakers Bureau; MSD: Consultancy, Other: Advisory board, Speakers Bureau; Beigene: Other, Speakers Bureau; Incyte: Other, Speakers Bureau; KYOWA KIRIN: Other, Speakers Bureau; EUSAPHARMA: Consultancy, Other, Speakers Bureau; ROCHE: Other, Speakers Bureau; SANDOZ: Other: Advisory board; NOVARTIS: Consultancy, Other, Speakers Bureau; ADC Therap.: Other; VERASTEM: Consultancy, Other: Advisory board, Speakers Bureau. Gordon: Zylem Biosciences: Patents & Royalties: Patents, No royalties; Bristol Myers Squibb: Honoraria, Research Funding.
duvelisib is a PI3K inhibitor indicated for the treatment of relapsed/refractory CLL/SLL and FL. The approved dose of duvelisib is 25mg BID. A cycle consists of every 28 days.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal